Part 2: Informed Consent
Conducting clinical trials requires a rigorous commitment to ethics and regulation to ensure participant protection and the validity of the results. In this context, informed consent emerges not merely as a bureaucratic requirement, but as the cornerstone of all ethical research involving human beings.
The Informed Consent Form (ICF) is an essential document that formalizes the participant's agreement to take part in the study, ensuring that their decision is made voluntarily, with full awareness and understanding. To be valid, according to Brazilian Resolution CNS N? 466/2012, the ICF must include clear and accessible information about the study's objectives, the procedures involved, potential risks and benefits, follow-up methods, available therapeutic alternatives, assurance of data confidentiality, and the participant?s right to withdraw consent at any time without penalty.
More than a formal requirement, informed consent is a continuous process that reinforces volunteer autonomy and transparency in the conduct of the research. In this sense, ensuring an ethical and well-structured ICF is essential for the credibility and acceptance of clinical trials.
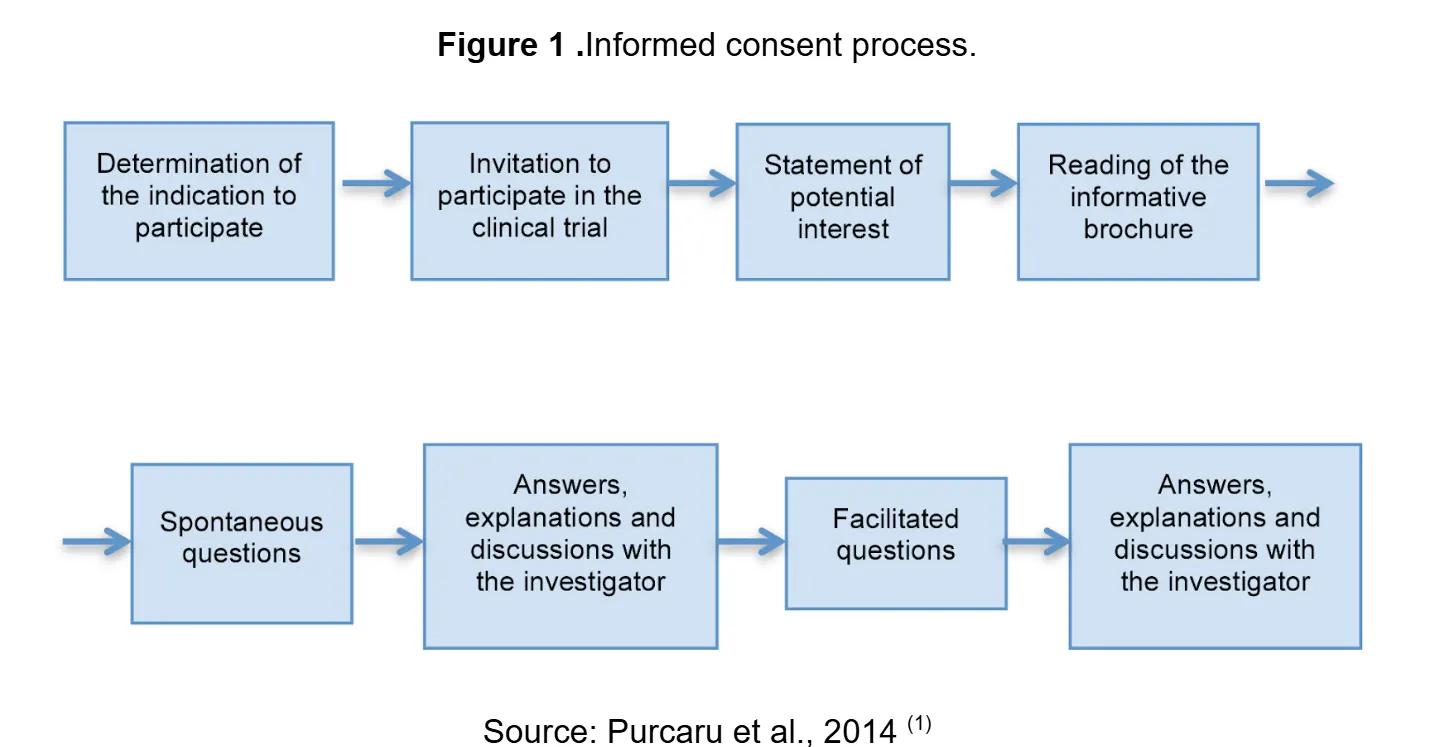
How does it work in practice?
Informed consent goes beyond signing a document.
Many still view informed consent as a form to be signed before a study begins. This simplistic view is far removed from the ethical reality that responsible researchers must implement. It requires providing complete and understandable information including all necessary elements such as purpose, procedures, risks, benefits, and alternatives using accessible language. Voluntariness must be guaranteed, ensuring the decision is free from coercion or undue influence. Comprehension must be taken into account, adapting the process for individuals with different educational levels or cognitive limitations. Finally, it is important to recognize that consent is an ongoing process, not a one-time event.