
Part 3: Local Committee and National Commission
In Brazil, the ethical review system operates hierarchically. Every research project involving human subjects must first be submitted to a CEP (Research Ethics Committee), which represents the local review body. The CEP is linked to an institution (such as a university or hospital) and is responsible for evaluating whether the research respects fundamental ethical principles. CONEP (National Commission for Research Ethics), on the other hand, serves as a higher-level body that coordinates and oversees the work of the CEPs through the CEP/CONEP System. Not all projects require direct evaluation by CONEP.
How does it work in practice?
Every research project involving human subjects must be reviewed by the CEP of the institution where it will be conducted. This is a mandatory rule in Brazilian research with no exceptions. However, not all projects need to be evaluated by CONEP. This national commission only reviews studies that fall within specific thematic areas, as defined by CNS Resolution 466/2012. These areas include:
- Research with foreign cooperation
- Studies involving new drugs, medications, vaccines, and diagnostic tests
- Research involving Indigenous populations
- Projects related to biosafety
- Studies on human reproduction
- Research in human genetics
- Projects that received a negative opinion from a CEP and are in the appeal phase
Approval and Oversight by Ethics Committees
Research Ethics Committees (CEPs) or Institutional Review Boards (IRBs) play a fundamental role. Submissions must be complete and transparent, providing all necessary documentation without omitting relevant information. Managing amendments is equally important, protocol changes must be submitted for re-approval when necessary. Periodic reports must be submitted, offering regular updates on the study?s progress. The notification of adverse events is critical, with serious and unexpected adverse events requiring prompt reporting. Serious adverse events must be reported to the CEP within 24 hours of their occurrence.
Finally, CEPs require continuous monitoring of research through interim reports, usually submitted semiannually or annually, as well as a final report at the end of the study. These documents allow the committee to verify whether the study is being conducted as planned and approved.
The submission process works as follows: the researcher registers the project on the Plataforma Brasil system and submits it to the CEP of their institution. If the project is approved by the CEP and falls under one of the special thematic areas, it is automatically forwarded to CONEP for a second review. This means that CONEP only evaluates projects that have already passed an initial review by a CEP. The flow of project submission to Ethics Committees is illustrated in Figure 1.
Figure 1. Ethical processing flowchart
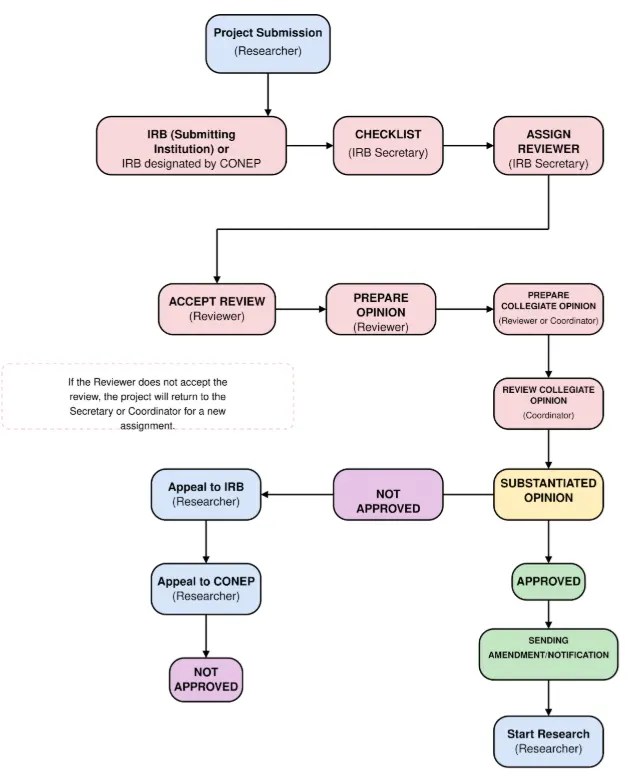
Source: Author