
Understand the Nuances that Drive Innovation and Safety in Medicine
--
Academic Clinical Study vs. Regulatory Clinical Study: What?s the Difference?
Clinical studies play a vital role in advancing medicine by generating scientific knowledge and validating new treatments. However, they can have different objectives and methodologies, generally classified as either academic clinical studies or regulatory clinical studies.
1. Academic Clinical Study
The primary purpose of an academic clinical study is to gain scientific knowledge, without necessarily aiming for the approval of a new drug or medical device. These studies are often conducted by universities, hospitals, and research centers, focusing on topics such as disease mechanisms, the effectiveness of existing therapies, or new clinical protocols.
Key characteristics:
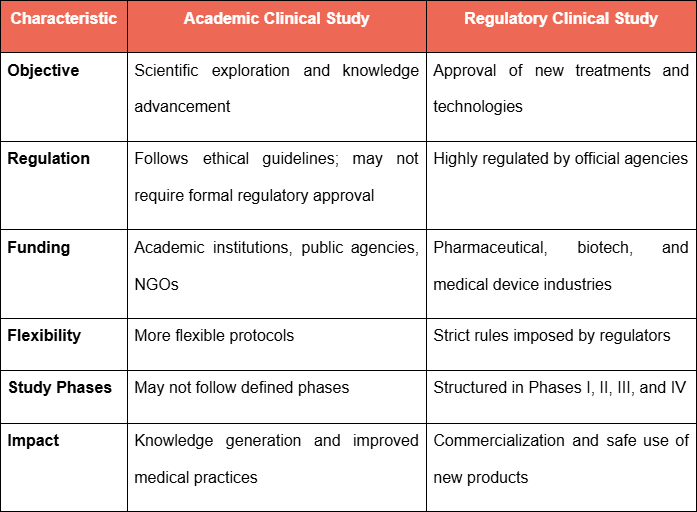
- Objective: To explore scientific hypotheses and deepen medical understanding.
- Regulation: Must follow ethical principles but may not require formal regulatory approval, depending on the study's nature.
- Funding: Typically supported by academic institutions, public research funding agencies, or non-profit organizations.
- Flexibility: Offers more freedom in designing protocols, since it does not need to meet the strict requirements set by regulatory agencies.

2. Regulatory Clinical Study
A regulatory clinical study aims to provide scientific evidence of the efficacy and safety of a new drug, medical device, or therapy to obtain approval from regulatory agencies, such as Anvisa (Brazil), the FDA (USA), or the EMA (European Union).
Key characteristics:
- Objective: To obtain regulatory approval for the marketing of a new product or therapy.
- Regulation: Must follow strict protocols established by regulatory authorities, such as Good Clinical Practices (GCP).
- Funding: Usually funded by pharmaceutical companies, biotechnology firms, or medical device manufacturers.
- Strict Protocols: Structured into phases, each with specific criteria to demonstrate the safety and efficacy of the product under investigation.
- Essential Documents: A range of documents must be produced before, during, and after the study, as required by regulations, to ensure data integrity.
Main Differences Between the Two Study Types
Conclusion
Both types of studies are essential for the advancement of medical science. While academic clinical studies are more exploratory and contribute to expanding scientific knowledge, regulatory clinical studies are crucial for ensuring the safety and efficacy of new treatments before they are introduced to the market. Understanding the distinction between them is key to recognizing their specific purposes and their impact on public health.
Contact us today by email: contact@grinn.co and find out how we can boost the success of your project.